Lesson 3 – Spectrophotometer and Micropipette use

Course: Life Science, Integrated Science, STEM
Unit: Ecological Networks Part 1- Network interactions
See Standards Addressed for all NGSS and WA State (Science, Math and Literacy). In addition to the aligned objectives linked above, for this lesson, here is a breakdown of:
- The purpose of the micropipette is to measure and dispense small volumes of liquids.
- A microliter (μL) is a unit of measure for small volumes of liquids in which 1 μL equals .000001 L.
- The proper use of a spectrophotometer including how to set the wavelength, measure the absorbance, and use a blank to zero the spectrophotometer between two readings.
- A reference (blank) is needed to zero (calibrate) the spectrophotometer.
- Optical density (O.D) is the unit used to measure absorbance.
- When the number of particles (food coloring) present in a given volume of the sample increases, the light absorbance increases too.
- Students use three different sizes of micropipettes to practice measuring given volumes of liquid using the proper technique (reading and setting the correct volume, measuring and dispensing intended volumes of a sample).
- Students use a spectrophotometer to measure the absorbance of various dilutions of food coloring and determine the relationship between absorbance and the number of particles in a sample.
Overview
The students will be introduced to two different pieces of equipment, the micropipette and the spectrophotometer. Both of these instruments will be used in a future investigation. First the teacher will introduce how to use both the micropipette and spectrophotometer through a short video clip and through modeling the use of the equipment. The class will then be divided into groups. Half of the class will start by completing the spectrophotometry activity and half of the class will start with the micropipetting activity. After about 20 minutes the groups will switch. Upon completion of the two activities, students will know how to use the equipment properly and efficiently.
Teacher Background Information: This activity is designed to introduce the students to micropipettes and spectrophotometers. Micropipettes are one of the primary tools of the laboratory biologist. These instruments allow you to measure and dispense small and accurate volumes of liquid solutions. Using correct pipeting technique will greatly increase the chance that your student’s experiment will return meaningful data so that their laboratory experience can be both fun and academically enriching. These micropipettes will allow you to accurately measure volumes as small as 2µl and as large as 1000µl. A microliter is 1 millionth of a liter or 10-6 L.
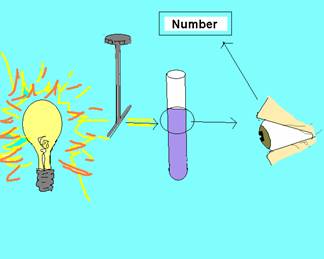
A spectrophotometer is a device used to measure light intensity. A spectrophotometer can measure either absorbance or transmittance of light. A small beam of light with a specific wavelength is emitted from the spectrophotometer which goes through the sample in a small glass container called a cuvette. The spectrophotometer measures how much light is absorbed by the sample or how much of the light passes through the sample which is transmittance. In this activity students will be using the spectrophotometer to measure the amount of light that is absorbed when various dilutions of food coloring are used. The more particles (food coloring) that are present in a given volume of a sample, the more of the light is absorbed and the less light that is transmitted. In the spectrophotometer activity, it is important to use yellow food coloring with the specified wavelength of 540 nm. The reason 540 nm is used with the yellow food coloring is because the wavelength of yellow is ~600 nm. In order to get a reading with the spectrophotometer, you need to set the wavelength at the complementary wavelength to yellow which is blue ranging ~500 nm. Given the specified food coloring concentrations, a wavelength of 540 nm has the best results. 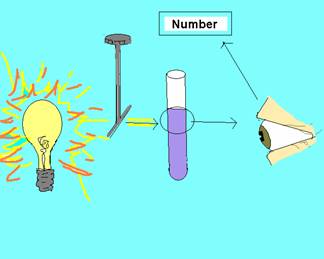

The light goes through an instrument that sets the wavelength. The small beam of light with a specific wavelength passes through the sample in a cuvette. Some of the light is absorbed and some of the light passes through. The amount of light that passes through is detected and a value is given by the spectrophotometer.
Before completing this lab, the teacher will need to check that they have all materials (see MATERIALS.doc) There is also some advanced preparation required (see ADVANCE PREPARATION.doc) in setting up the lab stations. The lesson is designed for 4 groups of 3-4 students to be working with the spectrophotometer and 6 groups of 3 students working with the micropipettes. The number of groups may vary depending on the number of students and the number of spectrophotometers available. Explain that half of the class will be begin working with the spectrophotometer and half the class will be working with the micropipettes. After about 20 minutes, the students will switch. Tell the students before the can complete the two activities you will be giving an overview of proper technique and procedure for both the micropipetting and spectrophotometer activity. A short video clip can be shown of a scientist using a micropipette in the lab (CSI or forensic science show). The teacher should emphasize that micropipettes are very expensive and need to be used properly to maintain the instrument and for accuracy in the lab. As a class, read the Objectives, Introduction and begin reading through the procedures. Have the students answer the questions on choosing the correct micropipette and setting the volume as you go through the procedures. When you get to Step 2 of Using the Micropipette, demonstrate each step at the front of the class. You may want to project the picture of the micropipette on the projector as well (micropipette.bmp). Be sure to emphasize the following as you demonstrate how to use the micropipette: Briefly go over the steps of the micropipetting activity. Be sure to emphasize the following when going over the micropipetting activity: Explain that the students will be working in groups of three. Each student will be using all three of the micropipettes. Project on the classroom screen, Micropipette Reminders.ppt, during the activity so students can be further reminded of the important steps. Explain that you are now going to go over how to use the spectrophotometer as a class. Read through the introduction to the spectrophotometer activity. Emphasize the picture showing how a spectrophotometer works. Explain that the light goes through a slit that sets the wavelength. The small beam of light with a specific wavelength passes through the sample in a cuvette. Some of the light is absorbed and some of the light passes through. The amount of light that passes through or the amount of light that is absorbed (this depends on the mode the spectrophotometer is set for, either absorbance or transmittance) is detected and a value is given by the spectrophotometer. Have the students read through the entire procedure. Be sure to emphasize that the spectrophotometer should be set to measure absorbance (not transmittance). Also emphasize that the graduations on the Beral pipets go up to 1 mL. Students will need to measure 1 mL four times in order to put the desired amount of liquid into the cuvette. Background Note for the Teacher: Students should observe that as the number of particles (food coloring) present in a given volume of the sample increases, the light absorbance increases too. Ask students why the cuvette with water needs to be inserted into the spectrophotometer between each reading. Emphasize the importance of a blank. Show the PowerPoint of what a blank is. First, ask students to explain how they would find the mass of a substance like salt using an electronic balance, a beaker, and salt. Students may say that you can put the beaker on the balance and then zero the balance or they may say that you can find the mass of the beaker alone and the mass of the beaker with salt. You can then find the mass of the salt by subtracting the mass of the beaker from the mass of the beaker with salt. Explain that determining absorbance of a sample is similar to finding the mass of a substance in a beaker. The substance that serves as the blank may absorb some of the light (not all the light may be transmitted). For example, in the activity the cuvette with water may absorb some light. In order to find the absorbance just the food coloring, you need to subtract the initial absorbance of the water from the new absorbance of the water and food coloring. Just like with an electronic balance, the spectrophotometer can be zeroed after the blank (cuvette with water) is placed in the spectrophotometer. Now when the cuvette with the water and food coloring is added, any change in absorbance is due to the addition of the food coloring not the water. ACTIVITY (Working in Groups). Assign students to work in groups of three. However, during the spectrophotometer activity, students may need to work in larger groups depending on the class size and the number of spectrophotometers available. After 20 minutes, the teacher should have the groups switch. The teacher may either want to collect all student work including the paper towel from micropipetting lab or have students attach filter paper to their lab journal. If you are not going to look at the paper towel right away, you may want students to draw a circle around each of their dried samples from the micropipetting activity. The smaller volumes become hard to see. Help students understand difficult vocabulary by breaking apart and explaining key root words. For example – spectrophotometer: spectro – photo – meter. “Spectro” comes from the Latin, specere, meaning appearance or to look at – it indicates a range or distribution to look at (e.g. What is the spectrum of trees around your school?). Even more simply, spect = look (e.g. spectacles, inspect). “Photo” means “light” and “meter” indicates something used to measure, often with numbers or quantities (quantitatively). When stringing root words back together, do so one step at a time. A photometer describes something that measures light. A spectrophotometer describes something that measures the range of light. Going even more in depth, this is a machine that measures the many wavelengths of light as they move through a liquid sample. You can go as deep as needed by your students. For example, to extend this further, explain that light is made of wavelengths and these wavelengths result in various colors. Since a spectrophotometer measures the amount of light and the range of light, or the wavelengths, it also measures color and intensity. As an extension, students can practice converting metric units. Examples are at the end of the student sheet for the micropipetting activity.INSTRUCTIONAL ACTIVITIES
INTRODUCTION (As a whole Group)